Ammonia Collection
"Unveiling the Creation of Ammonia: A Conceptual Journey through Chemistry" Step into the world of ammonia
All Professionally Made to Order for Quick Shipping
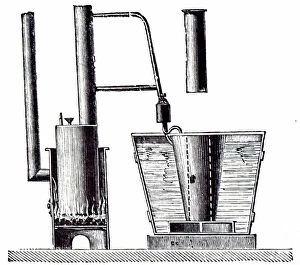
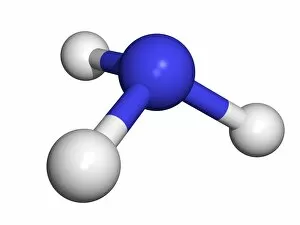
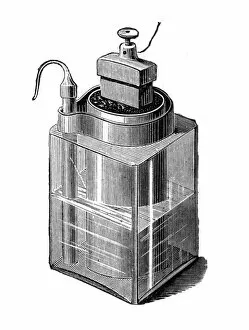
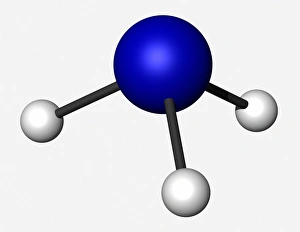
"Unveiling the Creation of Ammonia: A Conceptual Journey through Chemistry" Step into the world of ammonia, a remarkable compound that has shaped industries and revolutionized our understanding of chemistry. This captivating image takes us back to its origins, where Claude Louis Berthollet, a visionary French chemist, first discovered this invisible danger. The lithograph titled "The Invisible Danger" captures the essence of ammonia's potential hazards while showcasing its vibrant colors. It serves as a reminder that even though we cannot see it with our naked eyes, ammonia demands respect and caution. William Gossage's black and white photograph transports us to an era when pioneers like Henry Deacon and Holbrook Gaskell paved the way for industrial advancements in ammonia production. Their relentless efforts at oil shale works near Broxburn laid the foundation for future breakthroughs. As we explore further, NASA's mesmerizing image reveals Saturn's cloud deck – a celestial wonder reminiscent of the molecular structure itself. Just like this vast expanse in space, ammonia molecules bond together to create something extraordinary. Karl Bosch, a German chemist from the 1930s, enters the scene as he refines methods for large-scale production of ammonia. His contributions propelled industries forward by making this vital compound more accessible than ever before. Intriguingly enough, even advertisements such as Scrubbs bath lotion from 1934 recognized how by-products developed from coal by G. H. Davis could be transformed into valuable resources like ammonia. This highlights not only its importance but also its versatility across various sectors. Finally, let us marvel at the intricate beauty within an individual molecule – atoms intricately arranged to form bonds that hold immense power within their structure. Ammonia continues to shape our world today; it is used in fertilizers to nourish crops and plays a crucial role in refrigeration systems worldwide. Its creation remains an awe-inspiring testament to the ingenuity and perseverance of chemists throughout history.