Atomic Structure Collection
"Exploring the Depths of Atomic Structure
All Professionally Made to Order for Quick Shipping
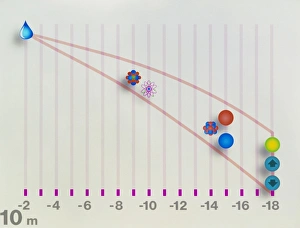
"Exploring the Depths of Atomic Structure: A Journey through Scientific Marvels" Step into the world as we delve into the groundbreaking discoveries made by brilliant minds such as Niels Bohr, Ernest Rutherford, and Erwin Schrodinger. In this captivating caricature, Niels Bohr stands tall, his genius shining through as he unravels the mysteries of atomic structure. His collaboration with E. Rutherford is beautifully depicted in another image where they are seen working together in a laboratory setting. Nuclear fission artwork takes center stage, showcasing the immense power hidden within atoms. This revolutionary concept was first introduced by Otto Hahn and Fritz Strassmann but further developed by Lise Meitner and James Chadwick. Rutherford's iconic caricature reminds us of his famous gold foil experiment that led to the discovery of a dense nucleus surrounded by electrons orbiting at specific energy levels. The electron structure of a helium atom is artistically portrayed, highlighting its two electrons occupying different shells. Erwin Schrodinger's contribution to quantum mechanics cannot be overlooked; his caricature symbolizes his pioneering work on molecular orbitals – regions where electrons are most likely to be found around atoms or molecules. The significance of these scientific breakthroughs lies in our understanding of atomic structures – how protons, neutrons, and electrons interact to form matter as we know it. Such knowledge has paved the way for advancements in various fields including chemistry, physics, medicine, and technology. As we marvel at these intricate depictions representing atomic structures from different perspectives throughout history - whether it be through artwork or laboratory scenes - let us appreciate how far humanity has come in unraveling nature's fundamental building blocks.