Covalent Bond Collection
"Unleashing the Power of Covalent Bonds: Exploring Graphene's Molecular Structure" In the realm of nanotechnology, one material reigns supreme - graphene
All Professionally Made to Order for Quick Shipping
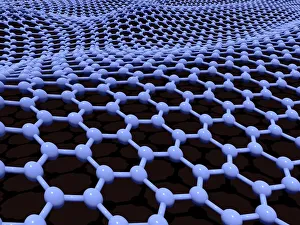
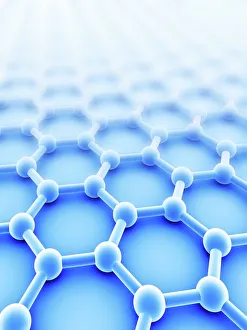
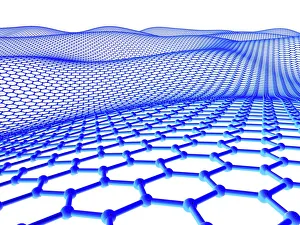
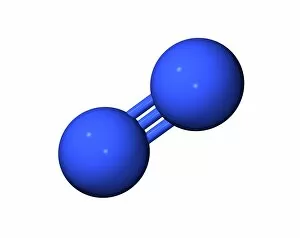
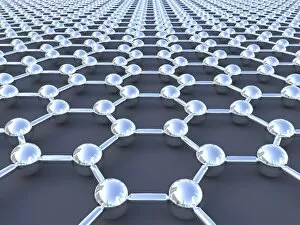
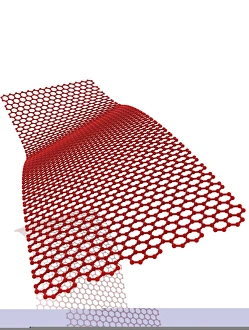
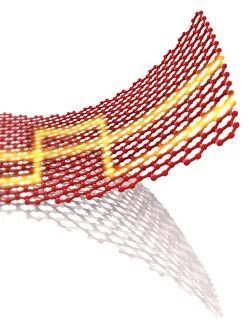
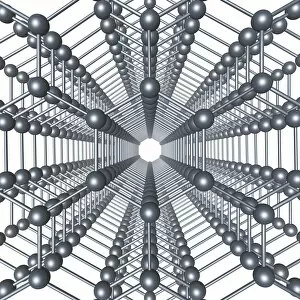
"Unleashing the Power of Covalent Bonds: Exploring Graphene's Molecular Structure" In the realm of nanotechnology, one material reigns supreme - graphene. This extraordinary substance, represented by its iconic hexagonal lattice structure, is composed of a single layer of carbon atoms tightly bonded together through covalent bonds. As we delve into the intricate world of graphene's molecular structure (C016 / 8518), we witness its remarkable properties unfold. Its ultrathin yet incredibly strong composition makes it an ideal candidate for various applications. From flexible electronics to advanced energy storage systems, this wonder material holds immense potential. The mesmerizing artwork C016 / 8274 showcases a graphene sheet in all its glory – a testament to the power and beauty that lies within these covalent bonds. Each carbon atom selflessly shares electrons with three neighboring atoms, creating an unbreakable network that defies conventional materials. But what exactly makes these covalent bonds so special? Let's zoom in on another captivating image (C016 / 8509) depicting multiple molecular structures of graphene. Here, we witness how each carbon atom forms four strong bonds with surrounding atoms, resulting in exceptional stability and conductivity. Nitrogen molecules also play a crucial role in enhancing graphene's properties. Their incorporation within the lattice structure further enhances its electrical performance while maintaining structural integrity (Graphene sheets, artwork). This synergy between nitrogen and carbon exemplifies the true essence of covalent bonding – a harmonious collaboration leading to groundbreaking advancements. Conceptual images portraying graphene conductivity (Graphene conductivity conceptual image) offer us glimpses into future innovations driven by these robust chemical connections. Scientists worldwide are harnessing this unique property to develop ultrafast transistors or even revolutionize solar cell technology. So next time you marvel at cutting-edge technologies or ponder over mind-boggling scientific breakthroughs, remember that behind it all lies the magic woven by covalent bonds.