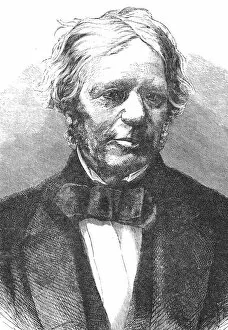Electrochemistry Collection
"Unveiling the Genius of Electrochemistry: Michael Faraday's Remarkable Contributions" Step into the world of electrochemistry
All Professionally Made to Order for Quick Shipping
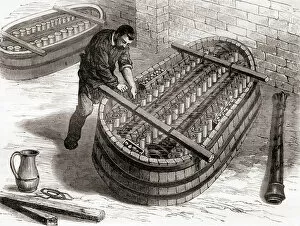
"Unveiling the Genius of Electrochemistry: Michael Faraday's Remarkable Contributions" Step into the world of electrochemistry, where scientific breakthroughs and innovation electrify our understanding of the natural world. At its core stands a brilliant mind, that of Michael Faraday, an English scientist whose legacy continues to illuminate our lives. In this captivating portrait by Charles Christofle, we catch a glimpse of Faraday's profound intellect and unwavering dedication to unraveling the mysteries of electricity. His pioneering work with batteries is beautifully depicted in "The Royal Institution electric battery, " showcasing his relentless pursuit for knowledge. Faraday's impact on electrochemistry cannot be overstated. Through meticulous experimentation and groundbreaking discoveries, he laid the foundation for modern-day electrical technology. This engraving captures his essence – a man driven by curiosity and armed with an insatiable thirst for knowledge. Born on September 22nd, 1791, Faraday's journey was one marked by resilience and determination. From humble beginnings as an apprentice bookbinder to becoming one of history's most influential scientists, his contributions revolutionized our understanding of electromagnetism. This coloured photograph immortalizes Faraday in all his brilliance – a testament to his enduring legacy. His tireless efforts paved the way for countless advancements in fields such as chemistry and physics. Gordon Ross' illustration further brings forth Faraday's remarkable achievements; it serves as a reminder that true greatness transcends time. Living Biographies aptly captures his spirit – an English scientist who forever changed our perception of science itself. As we delve deeper into the realm today, let us pay homage to Michael Faraday – a visionary whose indomitable spirit propelled humanity towards new frontiers. His name will forever be etched in scientific history as we continue to harness electricity for progress and enlightenment.