Electron Shell Collection
"Exploring the Intricate Dance of Electrons: Unveiling the Secrets of Electron Shells" In the vast realm of atomic structure
All Professionally Made to Order for Quick Shipping
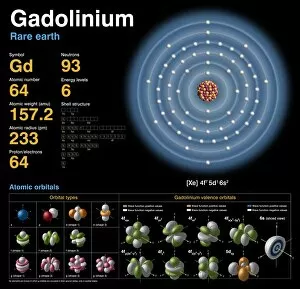
"Exploring the Intricate Dance of Electrons: Unveiling the Secrets of Electron Shells" In the vast realm of atomic structure, electron shells play a crucial role in defining an element's properties. Let's embark on a captivating journey through various atomic models and delve into their intricate arrangements. Beryllium, with its four protons and neutrons, showcases an elegant atomic model. Its two electrons gracefully orbit around the nucleus within their first shell, creating stability and balance. Helium, known for its noble nature, boasts a simplistic yet fascinating atomic model. With only two electrons occupying its single-shell configuration, it stands as a testament to stability and contentment. Moving forward to Boron's atomic model, we witness three valence electrons residing in different energy levels or shells. This arrangement contributes to Boron's unique chemical behavior and reactivity. Now let us shift our focus towards Rutherfordium—an element named after Ernest Rutherford himself—whose complex atomic structure captivates scientists worldwide. With multiple electron shells accommodating numerous electrons at varying distances from the nucleus, this enigmatic metal challenges our understanding of fundamental particles' behavior. Praseodymium enters the stage with its intriguing atomic structure—a spectacle that bewilders even seasoned researchers. Its 59 electrons occupy several distinct energy levels or shells surrounding the nucleus like celestial bodies dancing in harmony across space-time. As we revisit Rutherfordium once more—its significance cannot be overstated—we unravel further mysteries within its elaborate electron shell arrangement. The precise distribution of these subatomic entities determines Rutherfordium's chemical properties while leaving scientists awe-inspired by nature’s complexity. Phosphorus joins our exploration with an equally mesmerizing atomic structure featuring fifteen electrifying dancers encircling its phosphorous core. These energetic performers inhabit three separate shells showcasing Phosphorus' versatility when interacting with other elements. Within science books lie conceptual artworks illustrating these captivating phenomena—the dance between protons, neutrons, and electrons.