Cathode Collection
In the realm of technological advancements, one cannot overlook the significance of cathode
All Professionally Made to Order for Quick Shipping
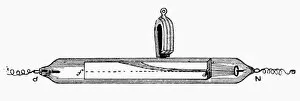
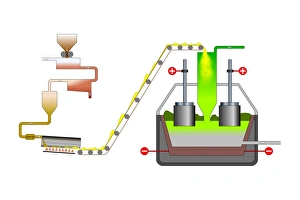
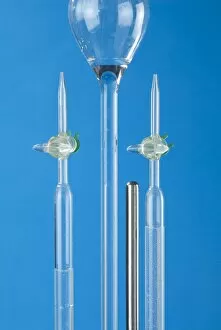
In the realm of technological advancements, one cannot overlook the significance of cathode. Dating back to 1949, this calculating machine revolutionized the way complex computations were carried out. With its intricate design and meticulous craftsmanship, it paved the way for future innovations in computing. But cathode's influence extended beyond just calculations. It played a crucial role in electrolysis of water, a process that split water molecules into hydrogen and oxygen through electrical energy. This breakthrough discovery opened up new possibilities in various industries such as fuel production and chemical synthesis. The mastery behind cathode was showcased in the finished electronic valves at Ernest Turner Electrical Instruments factory located in High Wycombe. The skilled artisans meticulously crafted these valves with precision and expertise, ensuring their flawless performance. Speaking of Ernest Turner Electrical Instruments factory, it served as a hub for innovation and excellence. Nestled amidst picturesque surroundings, this facility became synonymous with cutting-edge technology and groundbreaking inventions. Countless hours were spent here perfecting every detail to bring forth remarkable creations like cathode. As time passed by, Ernest Turner Electrical Instruments factory continued to thrive on its commitment to quality craftsmanship. Each day brought new challenges but also fresh opportunities to push boundaries further and redefine what was possible with cathode technology. From humble beginnings to becoming an integral part of scientific progress, it has left an indelible mark on history. Its legacy lives on through countless applications across diverse fields – from electronics to chemistry – shaping our modern world. So next time you encounter a calculating machine or witness electrolysis at work or marvel at electronic valves' functionality, remember that behind all these wonders lies the brilliance - an invention that changed everything we thought was possible.