5p electron orbitals
![]()

Wall Art and Photo Gifts from Science Photo Library
5p electron orbitals
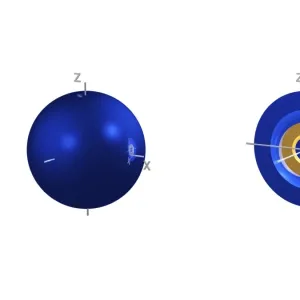
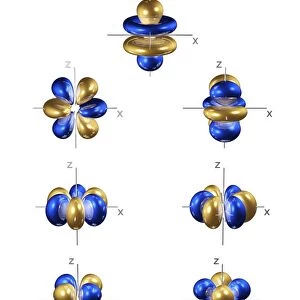
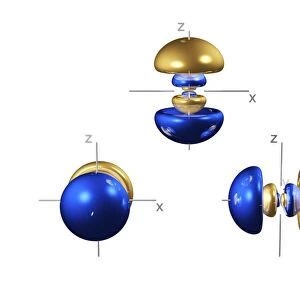
3p electron orbitals, computer model. An electron orbital is a region around an atomic nucleus (not seen) in which one or a pair of electrons is most likely to exist. The three 3p orbitals are made up of four lobes, two large outer ones and two smaller inner ones, centred on the nucleus. They are part of the 3 shell, which also contains a spherical, lower energy 3s orbital and five 3d orbitals at a higher energy (not seen). In atoms, electrons fill the lower energy orbitals first. The 3p orbitals are only filled when the lower energy 1s, 2s, 2p and 3s orbitals are full. Hence, the 3p orbitals are full in element 18, Argon (1s2 2s2 2p6 3s2 3p6)
Science Photo Library features Science and Medical images including photos and illustrations
Media ID 6283473
© DR MARK J. WINTER/SCIENCE PHOTO LIBRARY
Atom Atomic Axes Axis Configuration Electron Electron Orbital Mechanical Orbital Orbitals Quantum Mechanics Quantum Physics Shell Shells Trio Computer Artwork Physical
EDITORS COMMENTS
This print from Science Photo Library showcases the intricate beauty of 5p electron orbitals, specifically 3p electron orbitals, through a computer-generated model. Electron orbitals are regions surrounding an atomic nucleus where electrons are most likely to exist. In this image, the three 3p orbitals can be observed, each composed of four lobes - two larger outer ones and two smaller inner ones - symmetrically centered on the nucleus. These 3p orbitals belong to the third shell or energy level of an atom's electronic configuration. The third shell also encompasses a lower-energy spherical 3s orbital and five higher-energy 3d orbitals (not visible in this representation). As per the rules governing electron filling in atoms, lower energy orbitals are occupied before higher energy ones. Therefore, the 3p orbitals only become filled once all lower energy levels (1s, 2s, 2p, and 3s) have been completely filled. The completion of these fascinatingly shaped electron cloud formations occurs in element number 18: Argon. With its electronic configuration represented as "1s2 2s2 2p6 3s2". Argon has fully occupied its available lower-level shells before occupying its trio of symmetrical p-orbitals. This mesmerizing illustration combines elements from chemistry and physics to depict quantum mechanics at play within atomic structures. It serves as a testament to both scientific understanding and artistic interpretation while offering viewers a
MADE IN AUSTRALIA
Safe Shipping with 30 Day Money Back Guarantee
FREE PERSONALISATION*
We are proud to offer a range of customisation features including Personalised Captions, Color Filters and Picture Zoom Tools
SECURE PAYMENTS
We happily accept a wide range of payment options so you can pay for the things you need in the way that is most convenient for you
* Options may vary by product and licensing agreement. Zoomed Pictures can be adjusted in the Cart.