Orbitals Collection
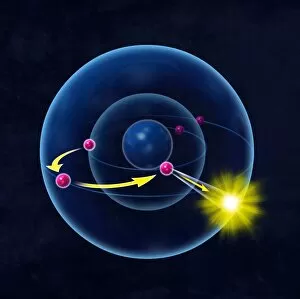
Orbitals: Unveiling the Quantum Dance of Atomic Structure In the realm of atomic structure, Niels Bohr's groundbreaking work revolutionized our understanding of orbitals
All Professionally Made to Order for Quick Shipping
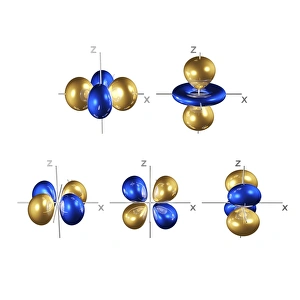
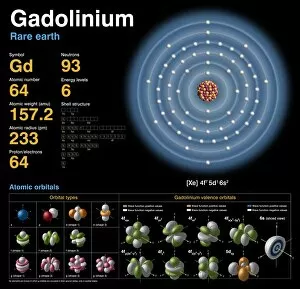
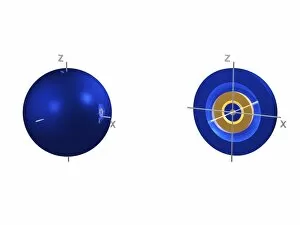
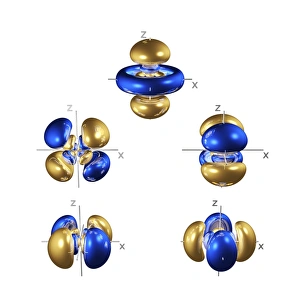
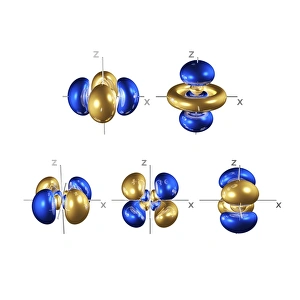
Orbitals: Unveiling the Quantum Dance of Atomic Structure In the realm of atomic structure, Niels Bohr's groundbreaking work revolutionized our understanding of orbitals. Often depicted in caricatures as a visionary scientist with his iconic model, he introduced the concept of quantised orbits resembling those observed in planetary motion. Delving deeper into this captivating world, we encounter electron orbitals that define the behavior and properties of quantum particles. Among them are the enigmatic 5f electron orbitals, forming a cubic set that adds complexity to atomic arrangements. These intricate patterns guide us through an invisible dance where electrons occupy specific regions around an atom's nucleus. Zooming further into this microscopic ballet, we discover another fascinating group - the 3d electron orbitals. They play a crucial role in shaping elements like Rutherfordium, contributing to its unique atomic structure. Named after Ernest Rutherford himself, this element embodies his pioneering spirit and relentless pursuit of unraveling nature's secrets. As we explore beyond Rutherfordium's boundaries, other elements like Praseodymium come into focus with their own distinct atomic structures. Each element presents its own symphony of electrons occupying various orbital configurations - an awe-inspiring testament to nature's infinite possibilities. The study of these mesmerizing orbitals not only deepens our knowledge but also fuels scientific advancements across numerous fields. From materials science to chemistry and beyond, understanding how electrons navigate these spatial landscapes unlocks new frontiers for innovation and discovery. So let us embark on this captivating journey through space at unimaginably small scales – exploring intricately woven paths where quantum particles gracefully move within their designated realms. In doing so, we continue to unveil the mysteries concealed within atoms while marveling at the elegance and complexity inherent in every aspect of our universe.